About geysers and other hot springs
When subsurface water comes into contact with volcanic heat, different kinds of hydrothermal features may be formed. The most spectacular ones are geysers. As in case of lava lakes in active volcanoes, the fascination of volcanic springs can partly be traced back to the understanding that they are a kind of umbilical cord which connects us to the planet's hot core, and moreover possibly also to the origin of life on earth. Geyser eruptions, furthermore, are primeval occurences taking place since the Earth's crust had cooled enough to host pools of liquid water in the Archean eon, 4,000 to 2,500 million years ago. So they may have been much more frequent in a distant past and remind us of geological eras long gone.
The ancient Greek philosopher Aristoteles would have described a geyser as "a synthesis of all four elements, water, earth, fire, and air". But it's unlikely that Aristoteles knew geysers, and even if he did, we better look for a more recent explanation.

How do geysers work?
Geyser activity has some in common with the railroad. Such as a train periodically transports passengers to a destination station, the water of a geyser transports heat from underground to surface at more or less regular intervals. Both, train or geyser, may or may not be on schedule. Transport by a geyser, in other words the eruption, starts if the collected amount of heat exceeds a trigger point. Now the steam pressure in the deep, hot zone is high enough to create steam bubbles, which need more space than liquid water and therefore push some water out of the geyser's orifice. The drop in hydrostatic pressure by discharging water causes more superheated water (i.e. liquid water above boiling temperature) to vaporize instantly, which in its turn jets out more water and so on. It's a very quick chain reaction, the lesser remaining water, the lower the pressure, the lower the boiling temperature, the more steam is generated and the more water is ejected. The eruption will continue until no more superheated water is left. Thus, a heat driven geyser works like an antiquated steam engine, whereby the water column corresponds to the piston.
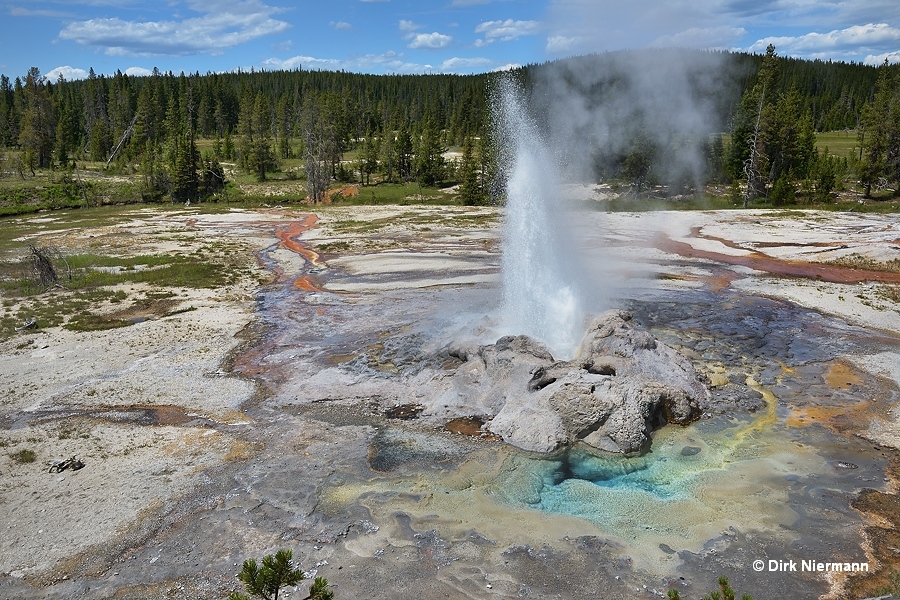
T. Scott Bryan (The Geysers of Yellowstone, 1995) defines a geyser as a spring, "characterized by intermittent discharge of water ejected turbulently and accompanied by a vapor phase (steam)." This definition holds for heat driven geysers, but can easily be transferred to cold-water geysers such as Geysir von Andernach, where the release of other gases than steam, mainly carbon dioxide, is the driving force. Both gases, steam and carbon dioxide, behave in exactly the same manner because just like steam bubbles also the formation of carbon dioxide gas bubbles increases with decreasing water pressure. It is the same effect that applies when opening a bottle of champagne.

The subterranean plumbing system represents the heart of a geyser. Ongoing reseach indicates that plumbing systems may feature different complexity, from mostly vertical, nearly unbranched conduits via conduits with bubble trap cavities alongside through to highly contorted, branched conduits with a lot of further sub-structures like fractures, fissures, constrictions, or porous spaces. Contorted piping systems may also have a large lateral extension. The one thing all plumbing systems have in common is a constricting vent near ground surface. Quite often different vents are interconnected by the same plumbing system, or different plumbing systems are interconnected and so geyser eruptions show dependencies between each other. Concerning Yellowstone, academic publications even suggest that all plumbing systems as well as all other thermal springs may be fed by a single giant aquifer in the deep. If this is true, and there really is evidence that it is, any interference with the system by geothermal drilling (even outside the park boundaries) could have desastrous consequences to Yellowstone's geysers.
A geyser eruption is predominantly characterized by height, discharge rate, duration, and interval, where interval stands for the time span between the starts of two subsequent eruptions. Generally speaking, the more isolated a geyser is, the more regular are its intervals. A good example of this is Old Faithful Geyser, even better examples are Riverside Geyser or Lone Star Geyser, all located in Yellowstone. On the other hand, interconnections between plumbing systems allow activity to shift and lead to more or less variable intervals. If a geyser suspends its eruptions for an unusually long period of time, which may be days or years depending on its former interval, it is called dormant. Whereas a geyser must be regarded as extinct if its plumbing system is irreversibly damaged, or has become sealed by geyserite deposits, or if the subterranean hot water supply has ceased. In other cases geysers were buried by a landslide. But in nature much is possible, so even geysers believed to be extinct may get a second life.
Are there different types of geysers?
Geysers are divided into two groups: Cone-type geysers and fountain-type geysers.
Cone type geysers eject water through a narrow vent with little or no surface pool. Often, but not always, the opening is embedded in a cone-type sinter structure. The eruption is characterized by steady water jets, which may be of impressive height.

Extremely elongated geyser cones have probably been formed on the bottom of lakes or seas.

Cone-type geysers may also have irregularly shaped vents, splitting the water jet up and diverting it in different directions.

In contrast, the eruption of fountain-type geysers is rising out of open pools and often shaped more like a dome than a jet.


Are all spouting springs regarded as geysers?
It is still a matter of controversy, if every intermittently spouting spring is a geyser, independently of the height of the eruption or the detectability of a rising vapor phase. If no rising vapor phase can be seen at all or gas/steam bubbles are rising without dragging major parts of the water column along with them, periodically spouting springs are classified as bubble-shower springs. In case an eruption occurs though no vapor phase is rising from the plumbing system, superheated water wells up in an open pool and the decrease in pressure causes violent boiling near the surface only.

In case of a poorly developed rising vapor phase, in some springs numerous small bubbles rise at the same time, while in others only a few but large bubbles appear. The "flashing" large steam bubbles out of Firehole Spring's vent are famous and gave rise to its name.


All in all, are bubble-shower springs true geysers? I think that the above-described variations are gradual expressions of one and the same "geyser effect". Somewhere in the water column the vapor pressure exceeds the pressure of the liquid water, a vapor phase forms and throws water above the previous surface. In my opinion it is decisive that it is a periodical effect, but not if it happens within the narrow sinuosities of the plumbing system or within the wider pool on top. In this regard a bubble-shower spring can be considered as geyser near utmost low temperature level.
And how does size matter? Well, if the eruption is only a gentle splashing, most observers wouldn't call that feature a geyser. Sometimes also the relation between the height of the eruption and the size of the pool or cone may influence the judgement. Every now and then geysers (for example Splendid Geyser in Yellowstone), which are capable of ejecting impressive water jets, are said to be dormant despite the fact that minor eruptions up to one meter / some feet height still occur. In all these situations a classification as active geyser may be correct, but doesn't match everyones expectation. Apparently, for those borderline cases the acceptance of an intermittently spouting spring as an active geyser lies in the eye of the beholder.

If temperature in the deep is just high enough to generate some trapped steam bubbles in the system, but not sufficient to induce periodical boiling of a bubble-shower spring, an external observer will only notice a intermittent rising and falling of the spring's water level, which also affects the overflow. This is due to expansions and contractions of the trapped steam bubbles. True intermittent springs never show any spouting activity or rising bubbles. Using the example of Doublet Pool, the time span between photo 1 and photo 2 was approximately 15 minutes. However, aside from intermittent hot springs there are also cold intermittent springs known, which probably work according to a siphon mechanism.

Also the constantly erupting or violently boiling perpetual spouters are not regarded as geysers. Bedrock with low thermal conductivity supports their formation because it leads to a low temperature difference (gradient) between water of deeper parts of the plumbing system and in the vent near ground surface. A perpetual spouter often is the extreme form of a geyser as opposed to a bubble-shower spring, featuring too high temperatures all along the subterranean tubing to let the systems water completely return into the liquid state. Therefore, if the temperature drops, a perpetual spouter may convert into a geyser, even if intervals remain very short in the range of seconds. So on location it is often hard to distinguish between a pulsating true perpetual spouter and a nearly perpetual spouting spring, which has to be classified as geyser.

A special type of permanently boiling spring can be observed with the so called "Frying Pan". This is wet soil or at most a very shallow pool, which is boiling extensively.

Finally, to put it straight and simple, if the amount of heat under ground increases steadily, a quiet spring may develop to an intermittent hot spring, then to a bubble-shower spring, next to a geyser, further to a perpetual spouter, and ultimately to a fumarole. The transitions between each stages are fluent.
Are there other types of hot springs?
In general, scientists distinguish between water-dominated and vapor-dominated hot springs, whether or not they are geysers. Water-dominated systems receive alkaline, chloride-rich or carbonate-rich waters from hot reservoirs in the depth, with some chemical changes occuring by water-rock interaction on the way to surface. On the other hand, in vapor-dominated systems hot, ascending volcanic gases such as hydrogen sulfide or gaseous hydrogen react with oxygen and groundwater and generate acidic fluids of higher sulfate concentrations, so-called acid-sulfate waters. Often the acidification is intensified by Sulfolobus archaea, which transform dissolved hydrogen sulfide into sulfuric acid. In many cases, the mere sight of a spring will be sufficient to tell whether it belongs to the alkaline or to the acidic water type.
The bright colors of crystal clear, alkaline pools have always fascinated observers. In contrast to cold waterbodies, floating green, brown, or red microscopic unicelled algae can't survive in most hot springs. Thus, their tint does no longer overlay the blue color generated by a combination of light scattering, the absorption of other than blue colors of the light and the intrinsic hue of pure water. In the process of light scattering preferentially the blue part of sunlight stimulates the oscillation of water molecules, which in their turn emit (scatter) blue light of the same color in all directions. This explains why the effect is most powerful in direct sunlight. The deeper a clear alkaline hot spring, the more intense also its brilliant blue tint.

By contrast, clear acidic springs in most cases display a blue-green hue because yellow sulfur deposits on their bottoms shine through. But attention should be paid not to confuse the egg yolk yellow thermophilic microorganisms on bottoms of alkaline springs with a sulfur lining, which usually appears paler.

The beige-green color of Champagne Pool in New Zealands Waiotapu is probably worldwide unique. It is caused by bottom deposits composed of red antimony pentasulfide and bright yellow orpiment (arsenic trisulfide), which add a beige tint to the pools slightly acidic, blue-green water. If you look carefully, you may spot in the photo also the sparkling gas bubbles, which together with the color of the water gave rise to the name Champagne Pool.

However, water of hot springs is not always transparent clear, but often clouded by suspended particles, thus forming a colloid. In particular acidic springs are quite regularly exhibiting floating, microscopic small mineral particles, here and there this is also found with alkaline springs. Turbidity is therefore no unmistakable proof, but a good first indication for acidic springs, especially if it appears to be gray, brown, yellow, yellowish green, orange or red. Such water clouding can be attributed to different processes. Besides the precipitation of formely dissolved minerals due to a temperature drop of the water near surface, also a simple washout of particles from soft soil by turbulences or by acidic decomposition may occur. Gray and bluish-gray particles often indicate sulfur compounds such as iron sulfide, yellow particles consist of pure sulfur, and red particles are often hydrated iron oxides.


Even more intensely colored by colloidal iron(III) oxide are the acidic Tomato Soup Pools in Yellowstone.

The probably most gorgeous visual effect comes from micro-particles consisting of pure silica. In this case light scattering generates beautiful bluish colors, which are changing with the view angle. Such springs, referred to as opalescent springs, are primarily alkaline ones but rarely also acidic ones. They feature somewhat lower temperatures and discharge rates, otherwise neither the precipitation nor the supply of silica from the underground would be sufficient to sustain the optical effect.

Some opalescent hot springs such as Blesi at Haukadalur in Iceland show the dependency of silica precipitation on the water temperature at its best.

An unusual variety of an acidic, particle rich spring is the "chocolate pot". Silica, iron oxides, and to a lesser extent other metal oxides are precipitated in form of a mound, which may reach an impressive height.

Quite rare is a permanent floating of larger particles, which are visible with the naked eye, on the water surface of hot springs. One example is Cinder Pool in Yellowstone National Park, where the black cinders are consisting of elemental sulfur and dispersed iron(II) sulfide. Particles on the surface of East Pyrite Pool in Lassen Volcanic National Park are mainly formed of pyrite (iron(II) disulfide).

Often the temperature of the ground is too high or there is not enough liquid water present to form clearwater or muddy hot springs. In this cases, penetrating vapor and hot gases are generating fumaroles. High gas pressure or high volumes are resulting in fumaroles with pronounced vents.

Many fumaroles emit a smell like rotten eggs, stemming from gaseous hydrogen sulfide, which reacts to elemental sulfur by contact with atmospheric oxygen. Accordingly, bright yellow sulfur deposits form around the vent. This kind of fumarole is also called solfatara. The reason is that the archetype of all sulfur lined fumaroles, the Bocca Grande, is located inside Solfatara volcanic crater on the Phlegraean Fields in Italy. However, recent science does no longer differentiate between both types but uses only the term fumarole.

Lower amounts of vapor or gases may also discharge through a field of barely visible pinholes, depositing very colorful minerals on the ground surface. Academic publications refer sometimes to it simply as "thermally altered ground".

Even submarine fumaroles are known from shallow waters around volcanic islands, for example at the hot water beach on the Aeolian Island Vulcano in Italy. In deeper waters gas emissions usually dissolve in the water before they can reach the surface.

Acidic gases emitted by fumaroles are capable of decomposing many types of soil. As a result a more or less highly concentrated slurry of very small degraded soil particles in a limited amount of acid is formed, called mud pot or mud pool.

For colorful, other than gray slurries also the term paint pot is used. Again, the differentation between muddy hot springs and mud pots is not sharp. Comparable to springs, mud pots may show different levels of activity, from a slight bubbling to intermittent bursts (mud geyser) to steady violent boiling.
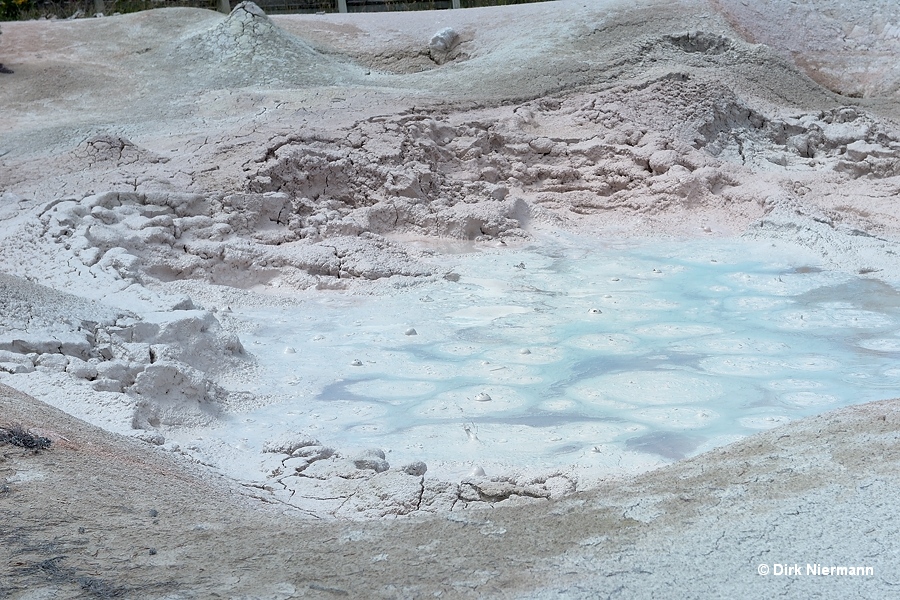
Spouting mud pots occasionally pile up material to the height of some meters in form of a more or less symmetrical mound. The ejected mud slides down the flanks of the cone and solidifies en route. Since this mechanism is comparable to lava flows, they are also referred to as mud volcanoes.

Content and photos on this homepage are protected by law. You may save photos on your Computer, but it is not allowed to use them in any other case without permission of the author. If you want to use photos please ask.